TRL 4
Looking for a partner interested in a license and/or a collaboration agreement to develop and exploit this asset.
Joven Maried, Jorge; Ras Mallorquí, María Rosa; Mariné Fortuny, Silvia; Maijó Ferré, Irene; Fernández Arroyo, Salvador; Rodríguez Gallego, Esther
11/03/2014
Application granted: 2016-07-07
The present invention relates to a method of in vitro diagnosis of non-alcoholic liver steatosis (NASH) comprising determining the concentration of alpha-ketoglutarate in a plasma or serum sample obtained from a subject, where a concentration of alpha-ketoglutarate greater than or equal to 3 {mi} M indicates that the subject suffers from NASH. Additionally, the invention relates to a method of in vitro diagnosis of non-alcoholic steatohepatitis, where a concentration of alpha-ketoglutarate greater than or equal to 20 µM in plasma or serum of a subject indicates that the subject suffers from non-alcoholic steatohepatitis. The invention also provides methods for deciding or recommending the administration of a suitable treatment for NASH or non-alcoholic steatohepatitis, as well as for establishing a subject’s response to treatment for NASH or non-alcoholic steatohepatitis.
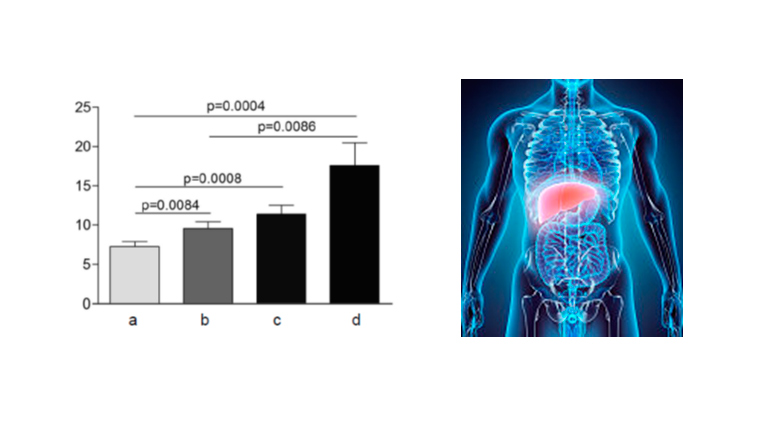
The inventors have found that alpha-ketoglutarate constitutes a plasma (or serum) biomarker with high clinical efficiency in the diagnosis of NASH. They have developed an in vitro diagnostic method of NASH that comprises determining the concentration of alpha-ketoglutarate in plasma or serum. The concentration of alpha-ketoglutarate increases significantly in patients compared to healthy controls (who do not suffer from NASH).
The clinical utility of the method developed by the inventors is good. The method provides a high sensitivity and specificity and far exceeds the clinical utility of other diagnostic methods of NAFLD already known in the state of the art, such as, for example, the determination of alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH). In addition, another advantage of the method of the invention is that it allows to determine the progression of the disease, since it has been found that increasing concentrations of plasma alpha-ketoglutarate are indicative of NASH in more advanced stages.
Therefore, another aspect of the invention provides a method for deciding or recommending the administration of a suitable treatment for non-alcoholic fatty liver disease or non-alcoholic steatohepatitis, which comprises the analytical determination of the concentration of alpha-ketoglutarate in the plasma or serum obtained from a subject, where if the concentration of alpha-ketoglutarate is equal to or greater than 3 10 µM, the start of treatment is recommended; in another hand, if the concentration of alpha-ketoglutarate is less than 3 µM, a clinical follow-up is recommended.
Application